by Joseph
Brazing Base Metals Containing Small Amounts of Titanium, Aluminum
 When nickel brazing Inconel 718 base metals in a vacuum furnace some of the difficulties experienced with the brazing include base metals that come out of the furnace dark and discolored, and the brazing filler metal doesn’t wet the surfaces well. In this article we will explain why this is happening, and what can be done about it?
When nickel brazing Inconel 718 base metals in a vacuum furnace some of the difficulties experienced with the brazing include base metals that come out of the furnace dark and discolored, and the brazing filler metal doesn’t wet the surfaces well. In this article we will explain why this is happening, and what can be done about it?
This is not an uncommon problem with a variety of base metals containing small amounts of titanium and/or aluminum. Both titanium and aluminum will easily oxidize, and once those oxides are formed they cannot be easily removed in a standard vacuum-furnace atmosphere. Yes, vacuum is an “atmosphere” in normal production environments since the level of vacuum in the furnace during typical brazing is such that there is, relatively speaking, a goodly number of air molecules still in the furnace, including moisture in that air. Of course, moisture represents the presence of oxygen, which can indeed react with either titanium or aluminum to form very tenacious titanium oxides and aluminum oxides on the surface of the base metal, which will inhibit or prevent brazing filler metal (BFM) flow. By Dan Kay
by George Vander Voort
Microstructure of Ferrous Alloys
 The microstructure of iron-base alloys is very complicated and diverse, being influenced by chemical composition, material homogeneity, processing and section size. This article offers a brief explanation of the terminology describing the constituents in ferrous alloys, and offers a basic review of steel microstructures.
The microstructure of iron-base alloys is very complicated and diverse, being influenced by chemical composition, material homogeneity, processing and section size. This article offers a brief explanation of the terminology describing the constituents in ferrous alloys, and offers a basic review of steel microstructures.
Microstructures of castings look different from those of wrought products, even if they have the same chemical composition and are given the same heat treatment. In general, it is easiest to identify heat-treated structures after transformation and before tempering. For example, if a mixed microstructure of bainite and martensite is formed during quenching, these constituents will become more difficult to identify reliably as the tempering temperature used for the product increases toward the lower critical temperature. Further, ferrous metallographers tend to use nital almost exclusively for etching, but nital is not always the best reagent to use to properly reveal all microstructures. By George Vander Voort
by Joseph
Vertical Test Specimen for Furnace Brazing
 Furnace brazing is a common brazing process around the world, and I have witnessed many brazing furnaces in action in many countries – from here to mainland China. Furnaces are convenient for brazing since the parts to be brazed can be easily loaded into a batch furnace or onto the belt of a continuous-belt furnace. The operator depends on the various furnace parameters (temperature, time, ramp rates, atmosphere controls, etc.) to ensure that the job of brazing each component will be done reliably, correctly and identically for each part that is subjected to those brazing cycles in that furnace.
Furnace brazing is a common brazing process around the world, and I have witnessed many brazing furnaces in action in many countries – from here to mainland China. Furnaces are convenient for brazing since the parts to be brazed can be easily loaded into a batch furnace or onto the belt of a continuous-belt furnace. The operator depends on the various furnace parameters (temperature, time, ramp rates, atmosphere controls, etc.) to ensure that the job of brazing each component will be done reliably, correctly and identically for each part that is subjected to those brazing cycles in that furnace.
An interesting question I have often encountered over the years with furnace brazers is this: “How do I know if a particular gap-clearance will work in my brazing furnace?” Please understand that each brazing furnace is unique and behaves in its own unique way. By this, I mean that even two furnaces of the same model number are not actually identical. Each one has its own personality, and the furnace operator needs to try to understand and work with each “personality.” By Dan Kay
by George Vander Voort
Metallography of Superalloys
 While specimen preparation of superalloys for metallographic examination is relatively straightforward, the metallographer must take into consideration some of the inherent characteristics of these complex alloys, such as high toughness, presence of large amounts of strengthening phase and high corrosion resistance, to ensure getting samples that clearly reveal their complex microstructures.
While specimen preparation of superalloys for metallographic examination is relatively straightforward, the metallographer must take into consideration some of the inherent characteristics of these complex alloys, such as high toughness, presence of large amounts of strengthening phase and high corrosion resistance, to ensure getting samples that clearly reveal their complex microstructures.
Superalloys are complex alloys of Fe-Ni, Ni-, and Co-base compositions. Their microstructure can be quite complex due to the potential for a variety of phases that can form in heat treatment or service exposure conditions. This article discusses the use of new metallographic materials to prepare these alloys and the different etchants required to reveal the structure of these alloys properly as a function of alloy composition, heat treatment and microstructural phases. This discussion is limited to iron-nickel and nickel-base alloys, but most of the comments are also applicable to Co-base alloys. By George Vander Voort
by Joseph
VAC AERO is launching a New Metallography Column with George Vander Voort!
 VAC AERO launches a Metallography column in the Redesigned issue of the What’s HOT! Newsletter!
VAC AERO launches a Metallography column in the Redesigned issue of the What’s HOT! Newsletter!
The new column, written by Metallography expert George Vander Voort will feature NEW or previously published articles from INDUSTRIAL HEATING magazine. The monthly articles will offer helpful Metallography applications, tips and techniques to commercial and captive heat treaters alike. George Vander Voort has a background in physical, process and mechanical metallurgy and has been performing metallographic studies for 43 years. George has consented to write articles on Metallography for VAC AERO since many of our clients will find this material quite useful in their daily operations. However, George’s willingness to provide this service to VAC AERO readers does not imply his specific endorsement of the company or its furnaces, but rather his desire to help the metal treating community to understand Metallography better. Read Entire Biography
by Joseph
Controlling Suppliers and Customers: Key to Successful In-House Brazing?
 Trying to braze materials from outside suppliers that are not compatible with your brazing process and trying to meet customer’s unrealistic specifications can create headaches in the braze shop that are not necessary, and can be alleviated by working closely with suppliers and customers to make effective brazing a shared goal.
Trying to braze materials from outside suppliers that are not compatible with your brazing process and trying to meet customer’s unrealistic specifications can create headaches in the braze shop that are not necessary, and can be alleviated by working closely with suppliers and customers to make effective brazing a shared goal.
Over the years, many brazing shops have experienced brazing problems (leakers and nonwetting surfaces, for example) due to “unknown” variables that crept into their brazing operations, resulting in the failure or rejection of many brazed assemblies. An evaluation of these situations often shows that some of the subcomponents of the brazements (such as brackets, fittings, etc.) come from outside suppliers, and the brazing shop is not aware of details of the manufacturing processes used by the suppliers to make the components that subsequently will be brazed. As a result, many shops are caught up in trying to fix the problem by trying to determine what is wrong with their own in-house brazing operations, often leading to frustration because no cause can be identified. In many such cases, this is due to incorrect assumptions about the sources of the problem. By Dan Kay
by Joseph
Reducing Metal-Oxides in Brazing – Part 2
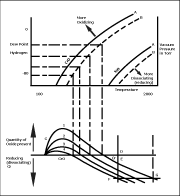 Let me make two important statements right at the start: 1. Surface-oxidation of metals will prevent effective brazing. 2. Brazing filler metals (BFMs) do not like to bond to, or flow over, oils, dirt, greases, or oxides on metal surfaces.
Let me make two important statements right at the start: 1. Surface-oxidation of metals will prevent effective brazing. 2. Brazing filler metals (BFMs) do not like to bond to, or flow over, oils, dirt, greases, or oxides on metal surfaces.
Thus, if any of the surface contaminants just mentioned are present on the metal surfaces to be brazed, effective brazing will not occur. Surface-oxidation is a common source of problems in commercial brazing. Parts to be brazed must be cleaned BEFORE assembling the parts for brazing, and then must be kept clean during the brazing process. One very effective tool that brazing engineers and shop personnel must understand and learn to use is the famous “Metal / Metal-Oxide Equilibrium Curves” published in 1970 in the AWS Welding Journal. By Dan Kay
by Joseph
Biography – Dan Kay
Dan Kay’s Biography Dan Kay (BMetEng, MBA), operates his own brazing consulting/training company, and has been involved full-time in brazing for more than 40-years. He received his Bachelor of Metallurgical Engineering degree from Rensselaer Polytechnic Institute in 1966, and his MBA from Michigan State University in 1982. Dan regularly consults in areas of…
by Joseph
Reducing Metal-Oxides in Brazing – Part 1
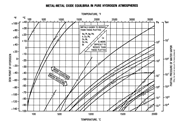 Let me make two important statements right at the start: 1. Surface-oxidation of metals will prevent effective brazing. 2. Brazing filler metals (BFMs) do not like to bond to, or flow over, oils, dirt, greases, or oxides on metal surfaces.
Let me make two important statements right at the start: 1. Surface-oxidation of metals will prevent effective brazing. 2. Brazing filler metals (BFMs) do not like to bond to, or flow over, oils, dirt, greases, or oxides on metal surfaces.
Thus, if any of the surface contaminants just mentioned are present on the metal surfaces to be brazed, effective brazing will not occur. Effective brazing requires the BFM to be able to alloy with (i.e., diffuse into) the base-metal being joined in order to form a strong, leak-tight metallurgical bond. The amount of alloying required is not large, e.g., copper BFM on steel actually alloys/diffuses much less than 5% and yet forms very strong, leak-tight brazed joints on steel. By Dan Kay
Next Month: In next month’s Part-2 article, we will look further into the interpretation and use of the metal/metal-oxide equilibrium-curves shown in Fig. 1, and describe a bit more about the oxidation/reduction reactions that may be occurring inside the brazing furnace throughout the brazing cycle.
by Joseph
Honeycomb-Brazing Essentials for Successful Use As Turbine Seals
 A honeycomb structure serves as an excellent gas flow seal and a sacrificial wear-surface to rotating turbine blades in high-temperature turbines. Achieving the ideal honeycomb construction requires careful attention to the amount and placement of brazing filler metal and brazing time and temperature.
A honeycomb structure serves as an excellent gas flow seal and a sacrificial wear-surface to rotating turbine blades in high-temperature turbines. Achieving the ideal honeycomb construction requires careful attention to the amount and placement of brazing filler metal and brazing time and temperature.
Honeycomb structures, one of nature’s unique designs, are widely used in such diverse applications as automotive, packaging, high-pressure containers, lightweight aerospace wing panels and engine nacelles, and high-temperature turbine seals for ground power and aircraft jet engines, taking advantage of honeycomb’s high structural strength with minimum weight. In the gas-turbine industry, honeycomb is used primarily in shaft-type labyrinth seals and rotating (rotor) blade shroud seals. This article focuses on the latter, and more specifically, on the use of open-face metallic honeycomb structures in high-temperature gas-turbine seal applications in aircraft jet engines and in industrial ground-power gas/steam applications. By Dan Kay